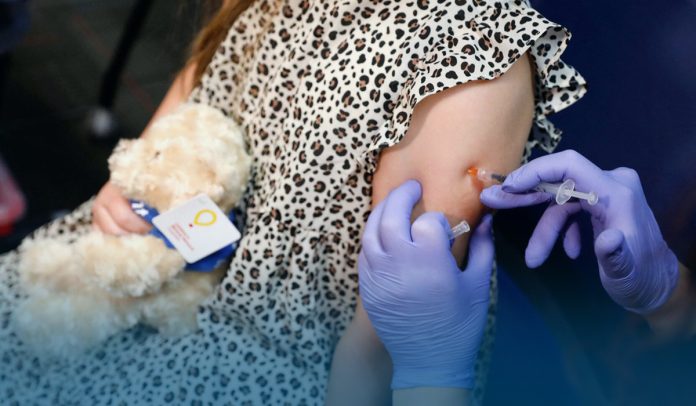
On Thursday, Moderna Inc, a Cambridge, Massachusetts-based US pharmaceutical and biotechnology company, said it requested U.S. FDA to approve its Coronavirus jab for kids below six years old, which would make it the first to offer low-dose shots for this age group.
The Coronavirus jab manufactured jointly by Pfizer Inc and its German partner BioNTech SE is approved for 5-year-olds and up. But their clinical trials for Americans 2-4-years showed lower antibody levels than in adults, driving the research to be pushed to test the 3rd shot. Manhattan, a New York-headquartered pharmaceutical giant, has stated that data would come in April.
Moderna files for emergency use for its Covid vaccine for children 6 months to 6 years.
They are saying 51% effective against symptomatic Covid for ages 6 months to 2 years and 37% for ages 2 to 6.
Sounds mediocre but here’s why it may be MUCH better than that….
🧵— Jeremy Faust MD MS (ER physician) (@jeremyfaust) April 28, 2022
Unsatisfied American families are waiting anxiously for an opportunity to defend the country’s youngest age group as people all around them discarded face coverings and other public health measures and procedures – although more virulent transformations of SARS-CoV-2 continue to transmit. Already nearly 75 percent of US children of all ages show signs of coronavirus infection in their bloodstream at some point during the deadly pandemic.
Moderna Inc submitted data to the US health regulator that it anticipates will demonstrate two low-dose vaccine shots can defend babies, toddlers, and preschoolers against the lethal virus – even though the productiveness was not nearly as high in children tested during the more infectious Omicron surge as earlier in the COVID-19 pandemic.
How much is a kid-sized dose effective?
According to Moderna, a biotechnology company that focuses on RNA therapeutics, two kid-sized shots were nearly 40-50% productive at avoiding symptomatic Coronavirus disease; not a home run for several parents of this age group, any defense would be better than none. Dr. Nimmi Rajagopal, MD, FAAFP of the Public health department in Chicago, Illinois, said that jab effectiveness is less than optimal. They’re anticipating for better usefulness, but this’s the foremost step.
In addition, Dr. Rajagopal of Cook County Health is impatiently waiting for immunizations for her littlest patients and her own three-year-old son. She added that it gives her such contentment to realize that hopefully, by Autumn, she’ll get her son in school, and he’d have received initial immunizations. And now, only kids 5-years and up could be inoculated in America, using competitor Pfizer/BioNTech’s jab, leaving eighteen million younger children unprotected.

Source: Web
Indeed, Moderna’s jab is not only the only one in the race. Pfizer Inc is soon likely to declare if three of its even lower-dose vaccine shots work for the youngest children, months after the disappointing sighting that two shots were not quite protective enough. Whether it is one Drug company’s doses or both, Dr. Peter Marks M.D., Ph.D., Director – Center for Biologics Evaluation and Research (CBER), said the federal health agency would move quickly without sacrificing their standards in determining if kids-sized shots are safe as productive.
Read Also: US CDC Says Around 60% of Americans Have Had COVID, Including 75% Children