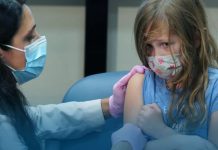
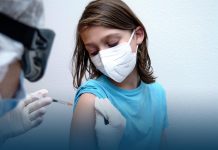
Pfizer, along with its partner BioNTech started an advanced level trial of their possible COVID-19 vaccines in volunteers in America. A spokesperson said that the health workers gave the dose to the first four participants on Monday at the University of Rochester Medical Center. The mutual Phase 2/3 trial, which looks for efficacy, safety, and ideal dosing of a drug or vaccine, will include about thirty thousand participants.
The first four participants were dosed Monday at the University of Rochester Medical Center. The combined Phase 2/3 trial, which looks for safety, efficacy and optimal dosing of a vaccine or drug, will include up to 30,000 participants. https://t.co/lMhwDhDHUX
— 4 News Now (@kxly4news) July 28, 2020
It was the second-high level trial of a coronavirus vaccine undergo in the United States on Monday. Earlier Monday, Moderna began its experimental vaccine Phase 3 trial in the United States. The vaccine of Pfizer is different, while it also uses an experimental approach that consists of genetic material to arouse an immune response against a key part of the COVID-19.
Earlier July, Pfizer, and BioNTech shared preliminary statistics showing that one of their vaccines, such as BNT162b1, appeared harmless and provoked antibody and T-cell immune responses in Phase 1 and Phase 2 trial. However, the company moved ahead with a somewhat different candidate. This vaccine will move forward in the combined Phase 2 and Phase 3, at around one hundred and twenty sites across the world, including thirty-nine states of the United States and Argentina, Germany, and Brazil.
Pfizer’s coronavirus vaccine uses pieces of genetic material
All COVID-19 vaccines of Pfizer use pieces of genetic material to provoke the vaccine receiver to make antibodies against some specific parts of the COVID-19. The first vaccine of Pfizer and BioNTech targeted a part of the spike protein, a structure that the virus uses to infect human cells. The second one, the Pfizer vaccine, moved into trials at the advanced level and targeted the whole spike structure.
Dr. Ugur Sahin, the founder of BioNTech, stated that this decision echos their key goal to bring a highly effective and well-tolerated vaccine to the market as early as possible. Whereas, they will continue to assess their vaccine candidates as part of a discriminated coronavirus vaccine portfolio. Chief adviser to the United States government’s coronavirus vaccine effort, Operation Warp Speed, Moncef Slaoui, said that tested COVID-19 vaccine platforms allow quicker development of vaccines while still being safe.
Slaoui says that they are selecting considerately and carefully vaccine technologies that are possible to work and work for the company. According to the drug giant, both types of Pfizer’s vaccine provoke antibody responses along with immune cells named T-cells. If the vaccine activates both arms of the body’s immune system, the hope is it will be more effective and give more strong protection.
In case of a successful phase 3 trial, Pfizer could supply 100 million doses by 2020 end
A Phase 1 study usually involves a small number of people and emphasizes whether a vaccine is harmless and provokes some type of immune response. However, in Phase 2, the clinical study extended to more people having the characteristics of the disease – such as physical and age health – similar to those for whom the vaccine intended, according to the United States Centers for Disease Control & Prevention. While in Phase 3, the health workers give the vaccine to thousands of people at more extensive scale and test the efficacy and safety of the vaccine.

Source: Web
If the vaccine trials become successful, Pfizer and BioNTech say they are on the right path to seek out the governmental regulatory review as sooner as October. Furthermore, if it receives emergency authorization from the United States Food & Drug Administration, it could supply up to a hundred million doses by the 2020 end, and around 1.3 billion by the 2021 end. Last week the United States Department of Health & Human Services, along with the Department of Defense, announced a $1.95 billion deal with Pfizer to make a hundred million doses of a coronavirus vaccine.
If the vaccine works, Pfizer and BioNTech say they’re on track to seek regulatory review as early as October https://t.co/GrrYd60y8O
— CNN Philippines (@cnnphilippines) July 28, 2020
According to the World Health Organization (WHO), the vaccine of Pfizer and BioNTech is one of twenty-five in clinical trials across the world. The Johns Hopkins University stated that over 4.2 million confirmed COVID-19 cases in the United States, and over 148,000 people lost their lives because of the virus.